ATP binding domain 4 Structures: Difference between revisions
Anharmustafa (talk | contribs) No edit summary |
Anharmustafa (talk | contribs) |
||
| (191 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
== General Properties == | |||
General information from PDB indicates that: | |||
(a) 1RU8 is a putative n-type ATP pyrophosphatase isolated from Pyrococcus furiosus, expressed in Escherichia Coli. | |||
( | |||
(b) Is a member of clan PP-loop | |||
(c) Resolution of 2.7 angstroms, with an r-value of 0.218. | |||
(d) Ligand chemical component identified as TRS (2-AMINO-2-HYDROXYMETHYL-PROPANE-1,3-DIOL). | |||
Information from SCOP when searched for 1RU8: | |||
1. Root: scop | |||
2. Class: Alpha and beta proteins (a/b) [51349] | |||
Mainly parallel beta sheets (beta-alpha-beta units) | |||
3. Fold: Adenine nucleotide alpha hydrolase-like [52373] | |||
core: 3 layers, a/b/a ; parallel beta-sheet of 5 strands, order 32145 | |||
4. Superfamily: Adenine nucleotide alpha hydrolases-like [52402] | |||
share similar mode of ligand (Adenosine group) binding | |||
can be subdivided into two group with closer relationships within each group than between the groups; the first three families | |||
form one group whereas the last two families form the other group | |||
5. Family: N-type ATP pyrophosphatases [52403] | |||
6. Protein: Putative N-type ATP pyrophosphatase PF0828 [102264] | |||
7. Species: Archaeon Pyrococcus furiosus [TaxId: 2261] [102265] | |||
== Surface Structure == | == Surface Structure == | ||
'''Pymol Visualization''' | |||
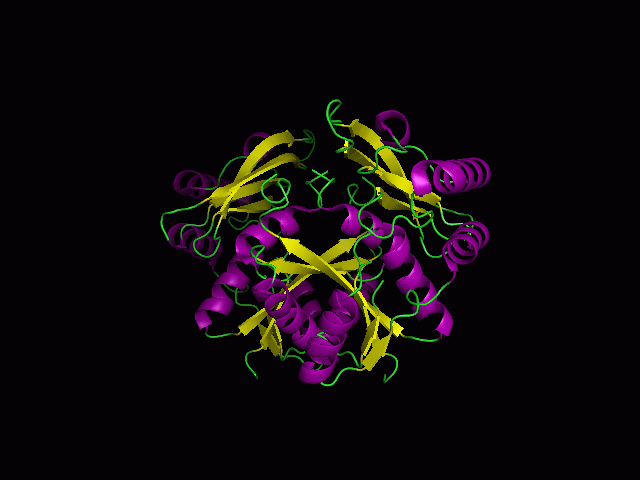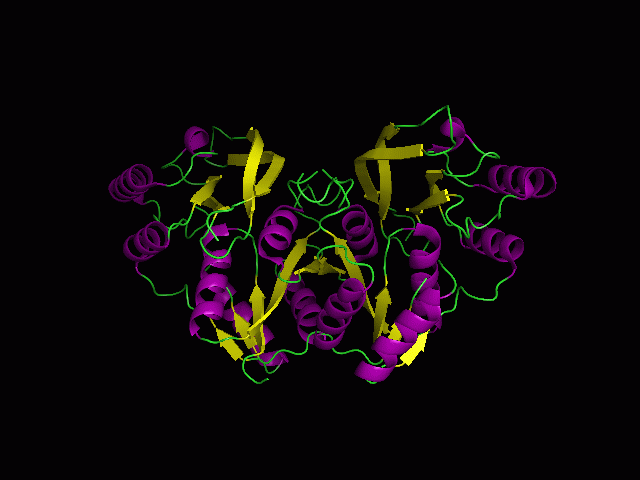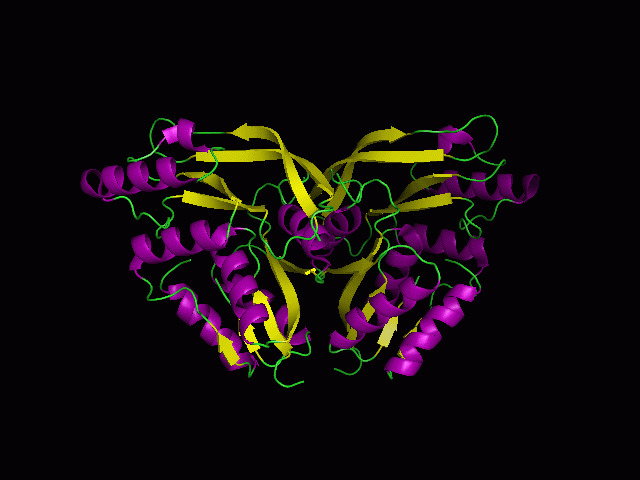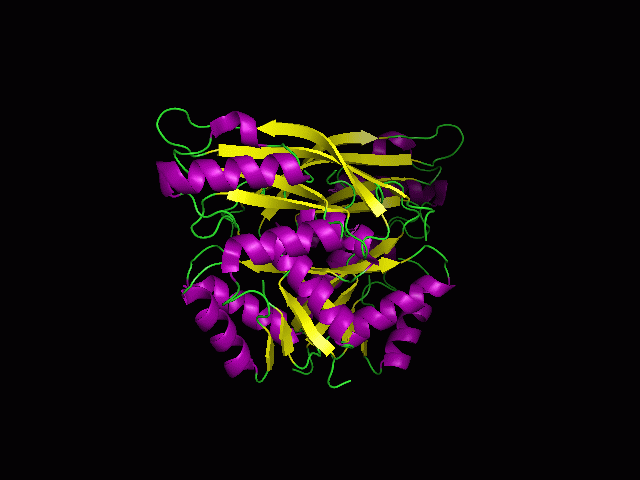
[[Image: | [[Image:1st.gif|left|thumb|700px|'''Figure 1.0'''. The three-dimensional structure of secondary structure 1RU8, as generated by PyMOL<BR> | ||
<BR> | |||
Purple- Helix<BR> | |||
Yellow - Sheet<BR> | |||
Green- Loop <BR>]] | |||
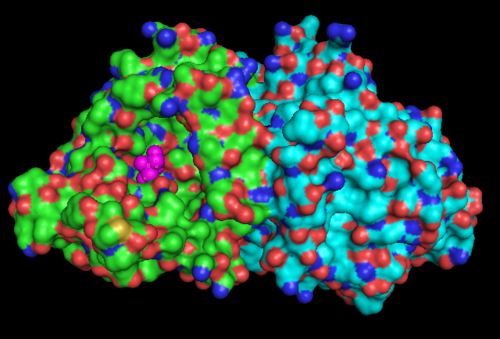
[[Image:1ru8.png|left|thumb|500px|'''Figure 1.1'''. The three-dimensional surface structure of 1RU8, as generated by PyMOL. | |||
Note that there is a artificial ligand, TRS (purple) bound to the protein]] | |||
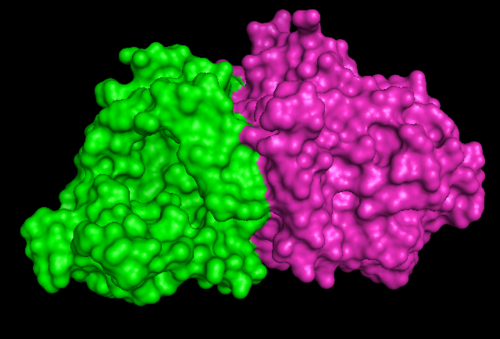
[[Image:1RU8 as 2 domain.png|right|thumb|500px|'''Figure 1.2'''. 1RU8 indicates its a dimer]] | |||
<BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR> | |||
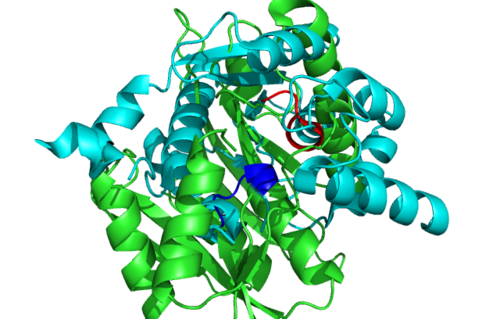
== Secondary Structure and Location of PP-loop == | |||
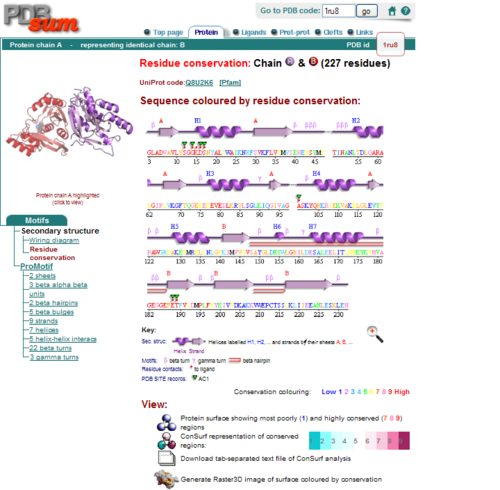
[[Image:1RU8 conserved residues.PNG|left|thumb|500px|'''Figure 2.0'''. Conserved residues of 1RU8 via PDBsum]] | |||
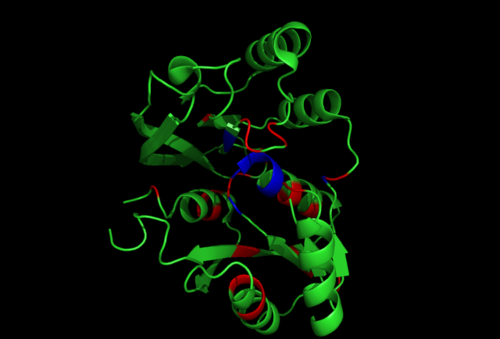
[[Image:1RU8-conserved region n interact with ligand.png|right|thumb|500px|'''Figure 2.1'''. Conserved residues of 1RU8 based on figure 4 visualized in Pymol. Highlighted red is the really conserved region and highlighted blue is the residues that is conserved and interact with the ligand ]] | |||
<BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR> | |||
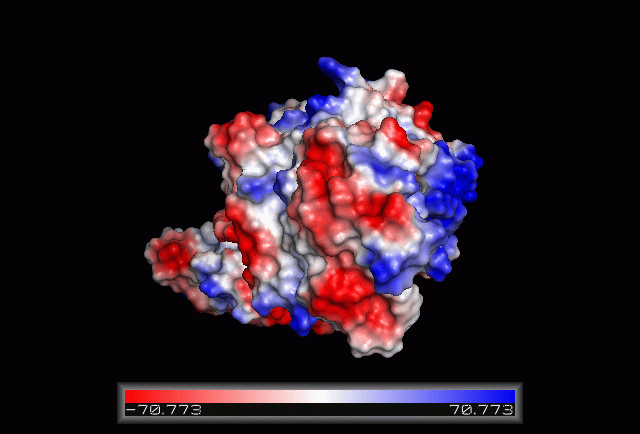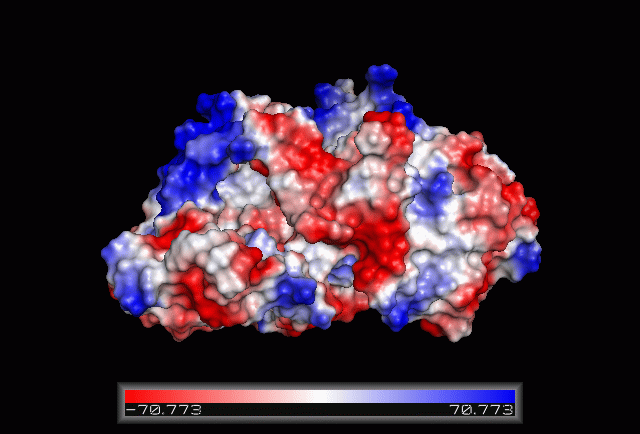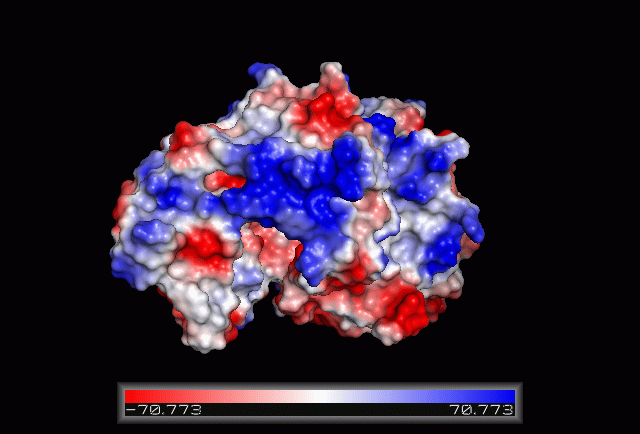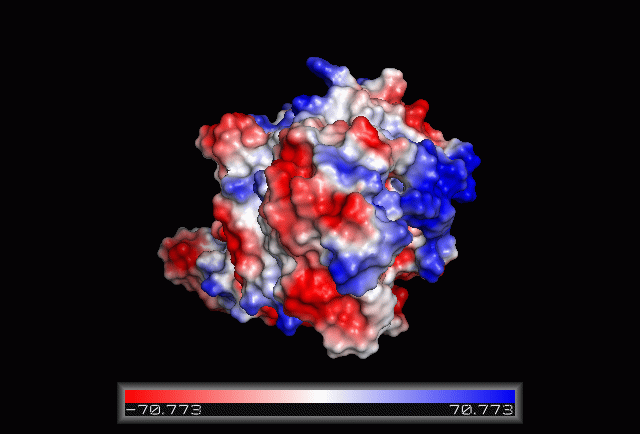
== Electrostatic Surface Potential == | == Electrostatic Surface Potential == | ||
[[Image: | [[Image:Electrostatic.gif|framed|none|'''Figure 3.0'''. The electrostatic surface potential. Blue colour indicated positive charge and red colour indicated negative charge surface whereas white colour indicated neutral charge]] | ||
== Structure Similarities == | |||
[[Image:Dali33.PNG|framed|none|'''Figure 4.0'''. The list of structural similarities to 1RU8 obtained using DALI. Highlighted by the box is the most similar structure that has known function which are used in this investigation.]] | |||
''Z score'' , the statistical significance of the similarity between protein-of-interest and other neighbourhood proteins. The program optimizes a weighted sum of similarities of intramolecular distances. | |||
''Root Mean Square Distance (RMSD)'', root-mean-square deviation of C-alpha atoms in the least-squares superimposition of the structurally equivalent C-alpha atoms. RMSD is not optimized and is only reported for information. | |||
''lali'', the number of structurally equivalent residues. | |||
''nres'', or the total number of amino acids in the hit protein. | |||
''%id'', percentage of identical amino acids over structurally equivalent residues. | |||
== Structure Alignment == | |||
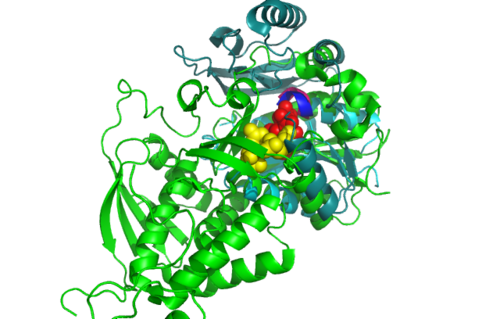
'''ATP binding Domain 4(1RU8) and Arginosuccinate Synthase (2NZ2)''' | |||
[[Image:Front view.png|left|thumb|500px|'''Figure 5.0'''. Structure alignment between 1RU8 and 2NZ2 generated via PyMol-Front view]] | |||
[[Image:Back view.png|right|thumb|500px|'''Figure 5.1'''. Structure alignment between 1RU8 and 2NZ2 generated via PyMol-Back view]] | |||
<BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR> | |||
Highlighted in green is the 2NZ2 while in magenta is the 1RU8. Since 1RU8 has 2 domain, domain B/2 is coloured darker in magenta for differentiation. There are 2 ligand indicated by the yellow (Citrulline) and red (ATP) spheres from 2NZ2. Highlighted also is the conserved region of PP-loop in pink and blue for 1RU8 and 2NZ2 respectively. | |||
<BR><BR> | |||
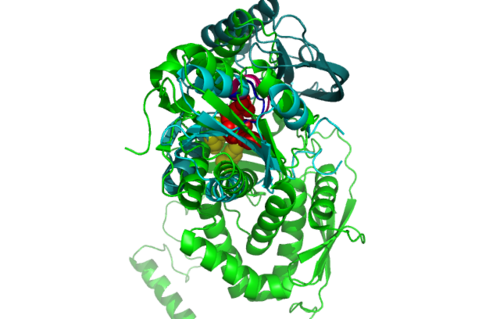
'''ATP binding Domain 4 (1RU8) and Queuosine Biosynthesis (3BL5)''' | |||
[[Image:1RU8 and 3BL5.png|left|thumb|500px|'''Figure 6.0'''. Structure alignment between 1RU8 (magenta) and 3BL5 (green) generated via PyMol. Highlighted colour of blue and red represent the conserved region of PP-loop.]] | |||
<BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR> | |||
<BR><BR> | |||
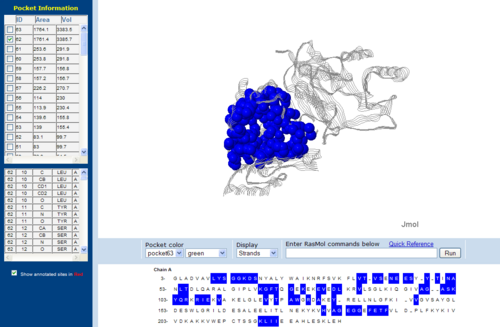
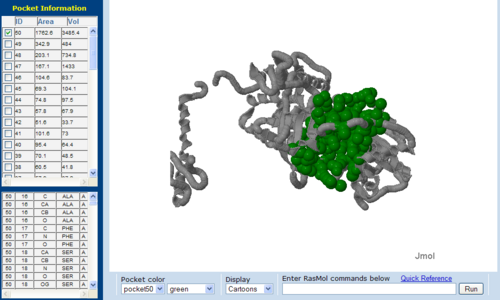
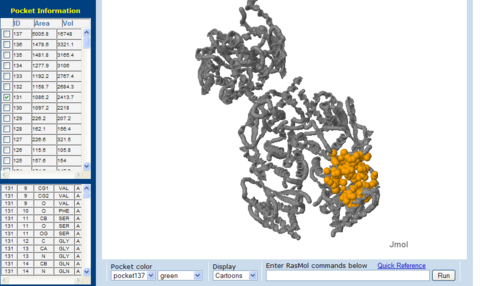
== Surface Cleft and Topography == | |||
[[Image:1RU8 | [[Image:Topo.PNG|left|thumb|500px|'''Figure 7.0'''. The topology of 1RU8 generated via CASTp]] | ||
<BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR> | [[Image:CAST-2nz2.PNG|right|thumb|500px|'''Figure 7.1'''. The topology of 2NZ2 generated via CASTp]] | ||
[[Image:CAST-3bl5.PNG|left|thumb|500px|'''Figure 7.2'''. The topology of 3BL5 generated via CASTp]] | |||
<BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR> | |||
The volume and size of the cleft of between protein indicated similarity between 1RU8 and 2NZ2. While 3BL5 has less similarity when compared to both 1RU8 and 2NZ2 | |||
<BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR> | |||
[[ | [[ATP binding domain 4 Abstract | Abstract]]| | ||
[[ATP binding domain 4 Introductions | Introductions]]| | |||
[[ATP binding domain 4 Methods | Methods]]|<BR> | |||
[[ATP binding domain 4 Structures | Structural Analysis]]| | |||
[[ATP binding domain 4 Functions | Functional Analysis]]| | |||
[[ATP binding domain 4 Evolution | Evolutionary Analysis]]|<BR> | |||
[[ATP binding domain 4 Discussions | Discussions]]| | |||
[[ATP binding domain 4 Conclusion | Conclusion]] | | |||
[[ATP binding domain 4 References | References]] | |||
[[ | [[ATP binding domain 4 | Back to Main ATP binding domain 4 pages]] | ||
Latest revision as of 08:38, 16 June 2009
General Properties
General information from PDB indicates that:
(a) 1RU8 is a putative n-type ATP pyrophosphatase isolated from Pyrococcus furiosus, expressed in Escherichia Coli.
(b) Is a member of clan PP-loop
(c) Resolution of 2.7 angstroms, with an r-value of 0.218.
(d) Ligand chemical component identified as TRS (2-AMINO-2-HYDROXYMETHYL-PROPANE-1,3-DIOL).
Information from SCOP when searched for 1RU8:
1. Root: scop
2. Class: Alpha and beta proteins (a/b) [51349]
Mainly parallel beta sheets (beta-alpha-beta units)
3. Fold: Adenine nucleotide alpha hydrolase-like [52373]
core: 3 layers, a/b/a ; parallel beta-sheet of 5 strands, order 32145
4. Superfamily: Adenine nucleotide alpha hydrolases-like [52402]
share similar mode of ligand (Adenosine group) binding
can be subdivided into two group with closer relationships within each group than between the groups; the first three families
form one group whereas the last two families form the other group
5. Family: N-type ATP pyrophosphatases [52403]
6. Protein: Putative N-type ATP pyrophosphatase PF0828 [102264]
7. Species: Archaeon Pyrococcus furiosus [TaxId: 2261] [102265]
Surface Structure
Pymol Visualization
Secondary Structure and Location of PP-loop
Electrostatic Surface Potential
Structure Similarities
Z score , the statistical significance of the similarity between protein-of-interest and other neighbourhood proteins. The program optimizes a weighted sum of similarities of intramolecular distances.
Root Mean Square Distance (RMSD), root-mean-square deviation of C-alpha atoms in the least-squares superimposition of the structurally equivalent C-alpha atoms. RMSD is not optimized and is only reported for information.
lali, the number of structurally equivalent residues.
nres, or the total number of amino acids in the hit protein.
%id, percentage of identical amino acids over structurally equivalent residues.
Structure Alignment
ATP binding Domain 4(1RU8) and Arginosuccinate Synthase (2NZ2)
Highlighted in green is the 2NZ2 while in magenta is the 1RU8. Since 1RU8 has 2 domain, domain B/2 is coloured darker in magenta for differentiation. There are 2 ligand indicated by the yellow (Citrulline) and red (ATP) spheres from 2NZ2. Highlighted also is the conserved region of PP-loop in pink and blue for 1RU8 and 2NZ2 respectively.
ATP binding Domain 4 (1RU8) and Queuosine Biosynthesis (3BL5)
Surface Cleft and Topography
The volume and size of the cleft of between protein indicated similarity between 1RU8 and 2NZ2. While 3BL5 has less similarity when compared to both 1RU8 and 2NZ2
Abstract|
Introductions|
Methods|
Structural Analysis|
Functional Analysis|
Evolutionary Analysis|
Discussions|
Conclusion |
References