Hypothetical protein Discussion: Difference between revisions
No edit summary |
No edit summary |
||
| (19 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
The hypothetical | The hypothetical putative sugar binding protein 2ob5 is part of the RbsD/FucU superfamily. It appears to be a FucU protein that catalyses the anomeric change of alpha-purine-fucose into beta-purine-fucose. This was found through combination of evolutionary, structural and functional analysis, and was then compared with existing literature. | ||
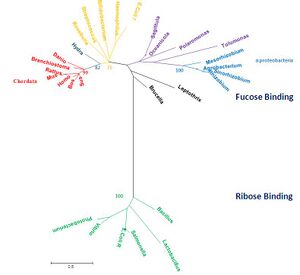
[[Image:Radiationtreexx22.jpg|left|thumbnail|Figure 1. Phylogenetic tree representing radiation, obtained using MEGA 4.0 [2] and manually edited in Microsoft Power Point 2007]] | |||
The MSA and tree diagrams showed a significantly divergent group of sequences that was completely separate from the remainder of the groups (green, bootstrap 100). This divergence was attributed to their being ribose transporters (RbsD). We therefore hypothesized that the gene encoding the RbsD transporter had undergone duplication in a common ancestor, as indicated by the presence of both RbsD and FucU(which are highly divergent) in the bacterium ''E. coli.''. It is believed that this duplication resulted the FucU paralog. In addition, Our query protein was found to have higher similarity to the FucU group of proteins than the RbsD. We did not encounter any RbsD transporter proteins in eukaryotes. | |||
The query protein was found in several alpha-proteobacteria (high similarity), supported by a bootstrap value of 100 (tree, blue). The chordate group was monophyletic (red, bootstrap value of 99), but sister group to several bacteria (yellow, bootstrap value of 75). We could not find any significant differences distinguishing these bacteria from the bacteria that were not sister group to chordates (purple).This raises a question about the evolution of our query: why do some bacteria have transporters that are more similar to eukaryotic proteins than to other bacterial ones? | |||
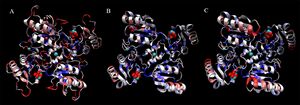
[[Image:2ob5_triple-set.jpg|right|thumbnail|Figure 5. Ribbon diagram showing similarities between 2ob5 (A), 1ogD (B) and 3e7n (C), coloured by BLOSUM60 of sequence conservation and shown with attached ligands]] | |||
Structural alignments found that the query protein matched well against RbsD transporter proteins. Structural analyses were limited by the fact that no fucose transporters had been crystallised at the time of writing. However, considerations were made during structural analysis to validate matching against RbsD proteins. Structural matches were found to form a decameric oligomer in a ring structure. This is an unnusual quaternary structure for a protein which is not membrane-bound (Kim et al. 2003). Close structural alignments and clear complementarities at subunit-subunit junctions suggest that 2ob5 is able to form this quaternary structure, although with some differences (Figure 5.). | |||
The binding pocket of a close match to 2ob5, 1ogD (RsbD protein found in ''B.Subtillius''), is clearly defined, consisting of Asp28, Tyr120, Glu122, His98 and Lys102 which coordinate a D-ribose. The binding cleft exists between two adjacent monomers, and a His20 residue from the neighboring monomer is highly conserved and involved in coordination. This suggests a critical role for the oligomerised state of the protein in ligand-binding. | |||
The 2ob5A binding pocket exhibits considerable similarity to the better characterised 1ogD, in that it has a corosponding His22 from adjacent monomers as well as matching Asp30 and Tyr136. A residue corresponding to Glu122 is notably absent, although this residue does not appear critical to ligand-binding (Kim et al. 2003). More significantly, the key His98 and Lys102 residues have been substituted for Arg114 and Tyr118 respectively. | |||
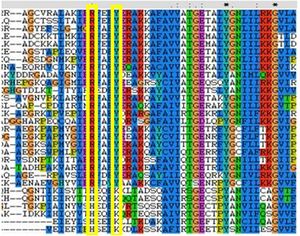
Comparison of these substitutions across the MSA found that these substitutions are highly conserved in all FucU proteins (see figure 2), while the His98 and Lys102 residues are strongly conserved in RbsD. | |||
[[Image:conres.jpg|right|thumbnail|Figure 2. This MSA shows the binding redidues that have been substituted in RbsD and FucU]] | |||
This is strong evidence that the query protein is a member of the FucU group of transporters. Previous studies have reiterated this point, associating the Arg/Tyr combination with Fucose-binding (Kim et al. 2003). This change in the binding pocket is likely to account for the difference in function between paralogs. | |||
The crystallographic structure of 1ogD also contains a metal-ion coordinating pocket formed by the interface between subunits adjacent between upper and lower sections of the ring. This ion – potentially a Cl - is cocrystallised in the structure of 1ogD, and appears to be coordinated by the Lys2 residue. This metal ion would have a potential stabilising effect on the oligomer. | |||
Visual characteristics of the corresponding 2ob5 region suggest that 2ob5 also exists as a decamer. However, the chloride-coordinating Lysine is replaced by an aliphatic leucine in 2ob5 and all other fucose-binding proteins analysed. From this, we hypothesise that the chloride ion is absent in fucose-binding proteins, but that these proteins are still able to assume a stable decameric ring. | |||
The functional analyses performed failed to give any major insight on the function of the target protein. The string analsyis only showed three possible interacting proteins, all of the interactions were inconclusive and failed to provide information about the protein. The Profunc results confirmed the fact that the target protein belongs to the FucU and RbsD superfamily. Unfortunally there were only two other members of this group that have had their structures crystallized and both are members of the RbsD group of transporters. This lack of homologs caused the profunc results to be inconclusive in all aspects. Another reason for this may be the fact that the crystal structure of our protein was monomeric, but according to structural analysis it was found to be oligomeric. | |||
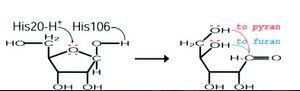
We then further investigated the role of RbsdD (ribose transporter) in comparison to our query. It was found that RbsD acts as a mutarotase to convert beta purine to beta furine. A proposed mode of action for this role of RbsD mutarotase can be seen in figure 3. | |||
[[Image:rbsfunction.jpg|left|thumbnail|Figure 3. Proposed Mode of action for RbsD]] | |||
The His20 residue protonates the O5 and the His106 deprotonates the OH attached to the C1. This causes ring-breakage allowing the formation of an equilibrium between the furan and pyran forms (Kim et al. 2003). The FucU is fundamentally different, changing the beta to alpha pyranse. The altered FucU residues in the binding site are thought to be the reason for the different functions. The two residues may cause the conversion of L-fucose by associating with the OH group at C-1 position of the sugar (Ryu, et al. 2007). | |||
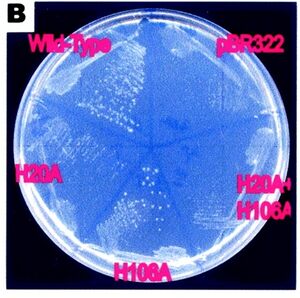
[[Image:petri.jpg|right|thumbnail|Figure 4. This shows the importance of the H-106 and the H-20 residues]] | |||
The importance of the H-20 and H-106 residues in the function of RsbD has been shown in an expereiment where these residues have been substituted for alanene. The Bacteria without H-20 residue had no pyrenase activity, and the residues without the H-106 lost appoximitly half of their activity. This was shown by growing them in a ribose substrate where the pressence of RsbD is necessary for the cell to survive (Kim et al. 2003)(see figure 4). | |||
Previous research has shown that RbsD proteins do not show any activity for L-fucose as a substrate, but that FucU proteins exhibit a limited pyranase activity for D-ribose. This is not unexpected, as the FucU protein still contains the functionally important H-20 residues (Ryu, et al. 2007). | |||
Latest revision as of 01:54, 16 June 2009
The hypothetical putative sugar binding protein 2ob5 is part of the RbsD/FucU superfamily. It appears to be a FucU protein that catalyses the anomeric change of alpha-purine-fucose into beta-purine-fucose. This was found through combination of evolutionary, structural and functional analysis, and was then compared with existing literature.
The MSA and tree diagrams showed a significantly divergent group of sequences that was completely separate from the remainder of the groups (green, bootstrap 100). This divergence was attributed to their being ribose transporters (RbsD). We therefore hypothesized that the gene encoding the RbsD transporter had undergone duplication in a common ancestor, as indicated by the presence of both RbsD and FucU(which are highly divergent) in the bacterium E. coli.. It is believed that this duplication resulted the FucU paralog. In addition, Our query protein was found to have higher similarity to the FucU group of proteins than the RbsD. We did not encounter any RbsD transporter proteins in eukaryotes.
The query protein was found in several alpha-proteobacteria (high similarity), supported by a bootstrap value of 100 (tree, blue). The chordate group was monophyletic (red, bootstrap value of 99), but sister group to several bacteria (yellow, bootstrap value of 75). We could not find any significant differences distinguishing these bacteria from the bacteria that were not sister group to chordates (purple).This raises a question about the evolution of our query: why do some bacteria have transporters that are more similar to eukaryotic proteins than to other bacterial ones?
Structural alignments found that the query protein matched well against RbsD transporter proteins. Structural analyses were limited by the fact that no fucose transporters had been crystallised at the time of writing. However, considerations were made during structural analysis to validate matching against RbsD proteins. Structural matches were found to form a decameric oligomer in a ring structure. This is an unnusual quaternary structure for a protein which is not membrane-bound (Kim et al. 2003). Close structural alignments and clear complementarities at subunit-subunit junctions suggest that 2ob5 is able to form this quaternary structure, although with some differences (Figure 5.).
The binding pocket of a close match to 2ob5, 1ogD (RsbD protein found in B.Subtillius), is clearly defined, consisting of Asp28, Tyr120, Glu122, His98 and Lys102 which coordinate a D-ribose. The binding cleft exists between two adjacent monomers, and a His20 residue from the neighboring monomer is highly conserved and involved in coordination. This suggests a critical role for the oligomerised state of the protein in ligand-binding. The 2ob5A binding pocket exhibits considerable similarity to the better characterised 1ogD, in that it has a corosponding His22 from adjacent monomers as well as matching Asp30 and Tyr136. A residue corresponding to Glu122 is notably absent, although this residue does not appear critical to ligand-binding (Kim et al. 2003). More significantly, the key His98 and Lys102 residues have been substituted for Arg114 and Tyr118 respectively. Comparison of these substitutions across the MSA found that these substitutions are highly conserved in all FucU proteins (see figure 2), while the His98 and Lys102 residues are strongly conserved in RbsD.
This is strong evidence that the query protein is a member of the FucU group of transporters. Previous studies have reiterated this point, associating the Arg/Tyr combination with Fucose-binding (Kim et al. 2003). This change in the binding pocket is likely to account for the difference in function between paralogs.
The crystallographic structure of 1ogD also contains a metal-ion coordinating pocket formed by the interface between subunits adjacent between upper and lower sections of the ring. This ion – potentially a Cl - is cocrystallised in the structure of 1ogD, and appears to be coordinated by the Lys2 residue. This metal ion would have a potential stabilising effect on the oligomer. Visual characteristics of the corresponding 2ob5 region suggest that 2ob5 also exists as a decamer. However, the chloride-coordinating Lysine is replaced by an aliphatic leucine in 2ob5 and all other fucose-binding proteins analysed. From this, we hypothesise that the chloride ion is absent in fucose-binding proteins, but that these proteins are still able to assume a stable decameric ring.
The functional analyses performed failed to give any major insight on the function of the target protein. The string analsyis only showed three possible interacting proteins, all of the interactions were inconclusive and failed to provide information about the protein. The Profunc results confirmed the fact that the target protein belongs to the FucU and RbsD superfamily. Unfortunally there were only two other members of this group that have had their structures crystallized and both are members of the RbsD group of transporters. This lack of homologs caused the profunc results to be inconclusive in all aspects. Another reason for this may be the fact that the crystal structure of our protein was monomeric, but according to structural analysis it was found to be oligomeric.
We then further investigated the role of RbsdD (ribose transporter) in comparison to our query. It was found that RbsD acts as a mutarotase to convert beta purine to beta furine. A proposed mode of action for this role of RbsD mutarotase can be seen in figure 3.
The His20 residue protonates the O5 and the His106 deprotonates the OH attached to the C1. This causes ring-breakage allowing the formation of an equilibrium between the furan and pyran forms (Kim et al. 2003). The FucU is fundamentally different, changing the beta to alpha pyranse. The altered FucU residues in the binding site are thought to be the reason for the different functions. The two residues may cause the conversion of L-fucose by associating with the OH group at C-1 position of the sugar (Ryu, et al. 2007).
The importance of the H-20 and H-106 residues in the function of RsbD has been shown in an expereiment where these residues have been substituted for alanene. The Bacteria without H-20 residue had no pyrenase activity, and the residues without the H-106 lost appoximitly half of their activity. This was shown by growing them in a ribose substrate where the pressence of RsbD is necessary for the cell to survive (Kim et al. 2003)(see figure 4).
Previous research has shown that RbsD proteins do not show any activity for L-fucose as a substrate, but that FucU proteins exhibit a limited pyranase activity for D-ribose. This is not unexpected, as the FucU protein still contains the functionally important H-20 residues (Ryu, et al. 2007).
Abstract | Introduction | Method |
Results | Discussion | Conclusion | References