DAP Functional Analysis: Difference between revisions
From MDWiki
Jump to navigationJump to search
No edit summary |
No edit summary |
||
| Line 25: | Line 25: | ||
---- | ---- | ||
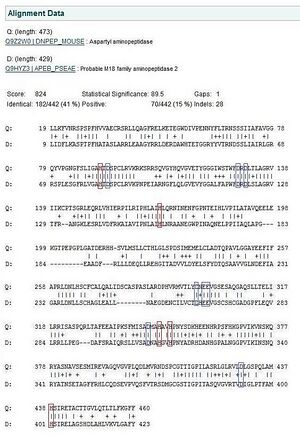
[[Image:mousepseudomonasalign.jpg| | [[Image:mousepseudomonasalign.jpg|thumb|'''Fig 4.7.''' A structural alignment between mouse and Pseudomonas show that all the residues that have been predicted to be involved in enzymatic activity have been conserved between the two species despite evolutionary distance.]] | ||
[http://compbio.chemistry.uq.edu.au/mediawiki/index.php/Aspartyl_Aminopeptidase]Return to aspartyl aminopeptidase | [http://compbio.chemistry.uq.edu.au/mediawiki/index.php/Aspartyl_Aminopeptidase]Return to aspartyl aminopeptidase | ||
Revision as of 05:44, 8 June 2008

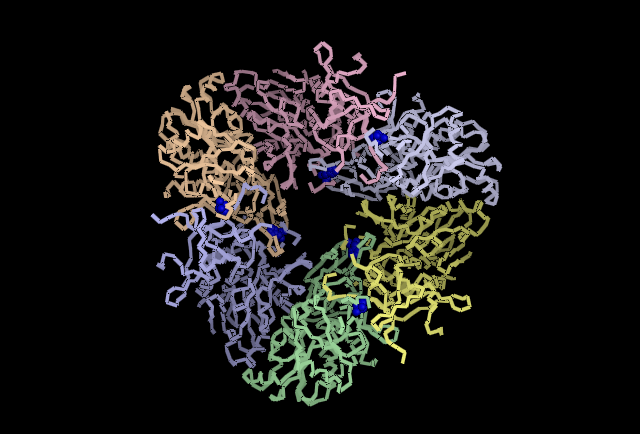
Fig 4.1. Shows an example from Wilk et al. (1998)[1], of how aspartyl aminpeptidase acts upon it's substrate.
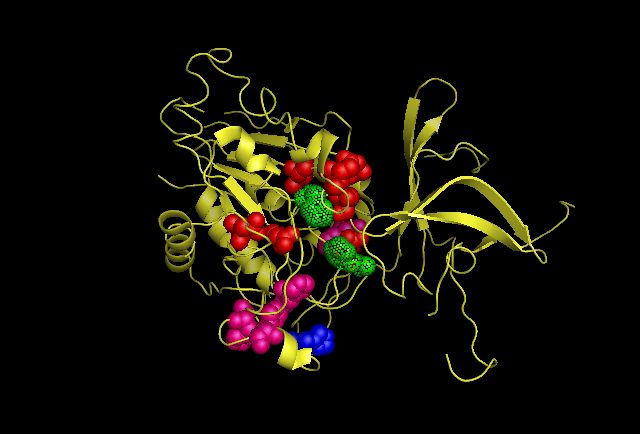
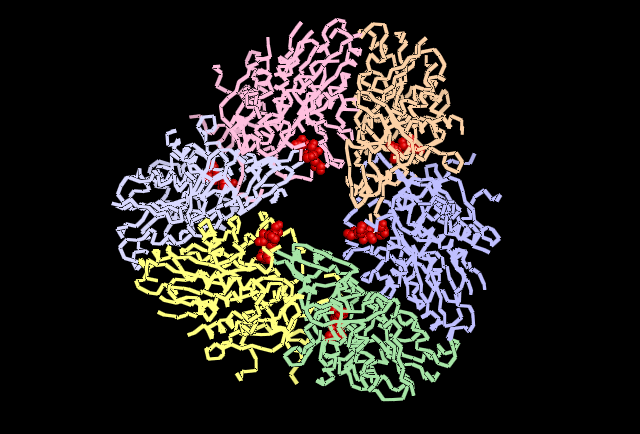
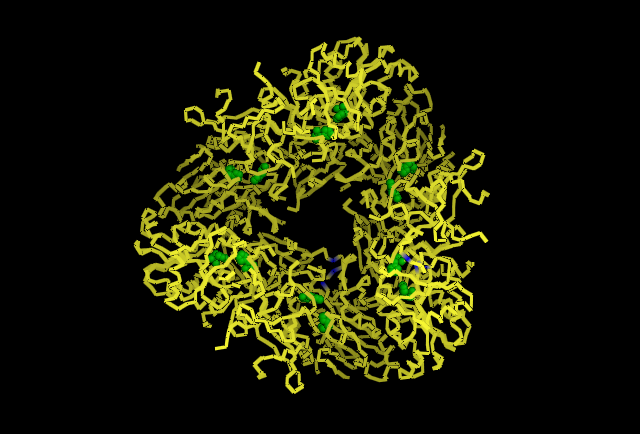
Fig 4.3. This image is chain A. Green shows the predicted active site residues His82 and His401. Pink shows Histidine residues that are also necessary for optimal enzymatic function, and experimentally mutations greatly reduce kcat. Blue is His313 predicted to be involved in subunit-subunit interactions. Red are Aspartyl and Glutamyl residues which are highly conserved across species and thought to participate in catalytic activity.
[2]Return to aspartyl aminopeptidase