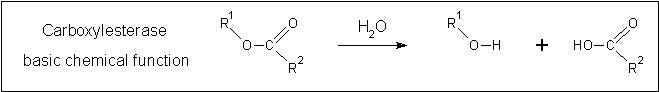Arylformamidase Discussion: Difference between revisions
Thomasparker (talk | contribs) No edit summary |
Thomasparker (talk | contribs) No edit summary |
||
| Line 16: | Line 16: | ||
Given that the phylogeny of our protein is largely consistent with traditional taxonomic groupings of organisms and that we can find no evidence of horizontal gene transfer, the delineations between prokariotic and eukaryotic species allow us to infer that the dominant mode of inheritance is clonal from bacteria to plantae and animalia. | Given that the phylogeny of our protein is largely consistent with traditional taxonomic groupings of organisms and that we can find no evidence of horizontal gene transfer, the delineations between prokariotic and eukaryotic species allow us to infer that the dominant mode of inheritance is clonal from bacteria to plantae and animalia. | ||
Further analysis revealed that 2pbl shared greater structural similarity with thermostable esterases of the HSL family compared to its other members. As discussed, thermostable proteins such as 2c7b often exist in dimers, a possiblity which remains uninvestigated for 2pbl. | Further analysis revealed that 2pbl shared greater structural similarity with thermostable esterases of the HSL family compared to its other members. As discussed, thermostable proteins such as 2c7b often exist in dimers, a possiblity which remains uninvestigated for 2pbl. | ||
Revision as of 13:49, 9 June 2008
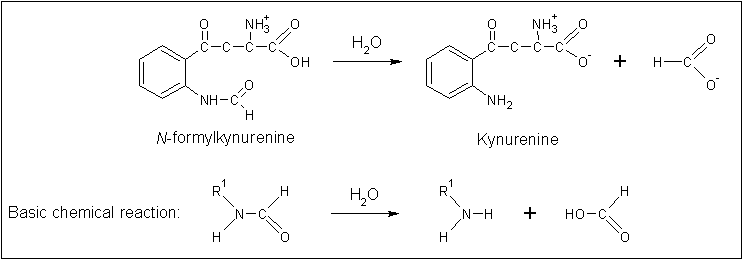
Functional inference from sequence similarity was restricted due to lack of supporting literature for highest-scoring sequences. Although the function of the arylformamidase from Mus musculus has been relatively well-characterised (Pabarcus 2005), its structure has not been determined. No arylformamidase proteins have not been characterised from amongst the prokaryotes, indicating it may be limited to the eukaryotes. Whilst a high-degree of conservation of the catalytic triad has been observed, there are proteins more structurally similar which provides a greater indication of function.
The carboxylesterase 2c7b was chosen for further analysis due to its high structural similarity to 2PBL. 2C7B is a thermostable carboxylesterase from an uncultured archaeon which was isolated in a thermal environmental sample (Byun 2007). This carboxylesterase belongs to a family of bacterial hormone-sensitive lipases/esterases (HSLs) known for their amino-acid sequence similarity to the mammalian hormone-sensitive lipase (Arpigny 1999). Despite the high structural similarity, the catalytic triad as identified in 2CB7 was not conserved in a sequence alignment. This may indicate that sequence alignment is not representative of structural similarities between 2CB7 and 2PBL. Structural alignment at these positions was not assessed.
There were a number of other bacterial HSL proteins as identified by Byun, et al. (2007) found to have a high structural similarity to 2PBL. Although a relatively low sequence similarity with 2PBL was observed, regions of conserved sequence characteristic to HSL proteins (Arpigny 1999) aligned with 2PBL. This may indicate that 2PBL is a member of the bacterial HSL family of lipolytic proteins. However, conservation of the catalytic triad within this alignment is still poor compared to the catalytic triad conservation between 2PBL and arylformamidase.
The HSL family encompasses a broad range of proteins with diverse functionality. They are characterised by their sequence similarity with the mammalian hormone-sensitive lipase. Interestingly, a mammalian hormone-sensitive lipase was not returned in the BLAST search, significant evidence against 2C7B being a member of the HSL family.
Clues to the function of 2PBL may be inferred from similarities between the fundamental reaction mechanisms of arylformamidases and carboxylesterases. Arylformamidase catalyses the hydrolysis of . Carboxylesterases catalyse the hydrolysis of a carboxylic ester into an alcohol and a carboxylic acid. Both involve hydrolysis, suggesting that 2PBL is indeed a hydrolase, an extremely broad enzymatic family. This does little to narrow down the specific function of 2PBL. Further analysis and experimentation is needed to refine this.
A multiple sequence alignment revealed several conserved regions in the sequence of 2PBL across all species. This high level of conservation was noted from the Bacteria through to the Eukaryota including vertebrates, invertebrates, yeasts and moulds. Most significantly, a relatively conserved catalytic triad consisting of serine, aspartimic/glutamic acid and histidine as well as many associated residues was observed in many of these sequences. The group of catalytic residues occurred in the same structural area of the protein. This may be indicative of a protein scaffold used ubiquitously in the evolution of hydrolases.
Given that the phylogeny of our protein is largely consistent with traditional taxonomic groupings of organisms and that we can find no evidence of horizontal gene transfer, the delineations between prokariotic and eukaryotic species allow us to infer that the dominant mode of inheritance is clonal from bacteria to plantae and animalia.
Further analysis revealed that 2pbl shared greater structural similarity with thermostable esterases of the HSL family compared to its other members. As discussed, thermostable proteins such as 2c7b often exist in dimers, a possiblity which remains uninvestigated for 2pbl. biological implications/applications...
Potential thermostability - applicability in industry.
further research...