Arylformamidase Additional Materials
Links
Protein Data Bank Entry for 2PBL
FASTA Sequence
>gi|146387357|pdb|2PBL|A Chain A, Crystal Structure Of Putative Thioesterase (Yp_614486.1) From Silicibacter Sp. Tm1040 At 1.79 A Resolution GXELDDAYANGAYIEGAADYPPRWAASAEDFRNSLQDRARLNLSYGEGDRHKFDLFLPEGTPVGLFVFVH GGYWXAFDKSSWSHLAVGALSKGWAVAXPSYELCPEVRISEITQQISQAVTAAAKEIDGPIVLAGHSAGG HLVARXLDPEVLPEAVGARIRNVVPISPLSDLRPLLRTSXNEKFKXDADAAIAESPVEXQNRYDAKVTVW VGGAERPAFLDQAIWLVEAWDADHVIAFEKHHFNVIEPLADPESDLVAVITA
File:Carboxylesterase (archaeon).txt PDB File:Carboxylase.txt PDB
Human arylformamidase has the following catalytic activity.
N-formyl-L-kynurenine + H2O → Formate + L-kynurenine
Required for elimination of toxic metabolites
It belongs to the AB hydrolase super family.
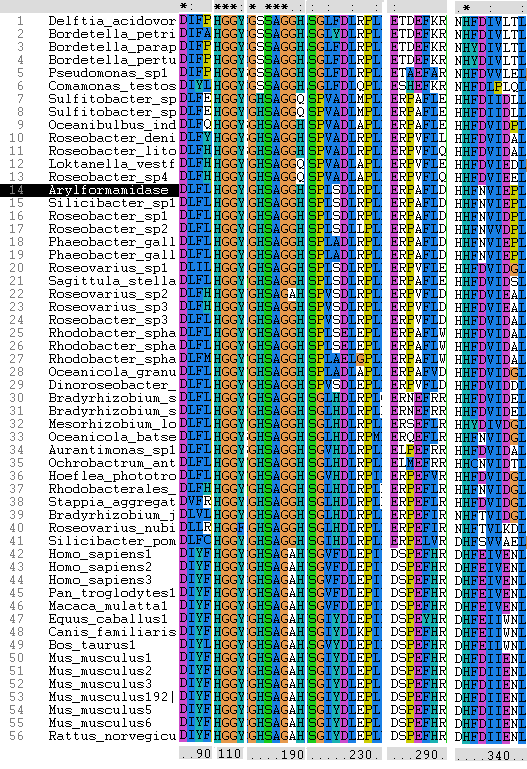
Even though the structure of the human protein hasn’t been determined, looking at the sequence alignment the catalytic triad residues are conserved. The residues have resisted mutation indicating that they are important for activity.
Our protein is similar to carboxylesterase which also belongs to AB suberfamily.
Each of the residues are linked to a turn region. The catalytic triad in Archaeoglobus fulgidus Carboxylesterase is very close to the ligand (see figure 12).
Sequence alignment:
Sections of alignment showing the conserved residues across bacterial and eukaryotic species.