Arylformamidase Structure
Methods
The structure of arylfromamidase was obtained from RCSB Protein Data Bank (PDB ID: 2PBL). http://www.rcsb.org/pdb/home/home.do
The predicted interaction arylformamidase with other proteins was determined using the STRING database (STRING: Search Tool for the Retrieval of Interacting Genes/Proteins). http://string.embl.de//
The DALI database was used for the structural comparison of arylformamidase with other proteins. http://ekhidna.biocenter.helsinki.fi/dali_server/
PDBsum database was used to determine the secondary structure of arylformamidase. http://www.ebi.ac.uk/pdbsum/
Results
Structure of Arylformamidase
Arylformamidase (All Chains)
The image above shows the chains A (upper right), B (upper left), C (lower right) & D (lower left) interacting. The molecules in the middle of chains A & B and chains C & D is phosphate ion (PO4). The green molecule between chain B & D is a magnesium ion (Mg). These ions aren't biologically significant and could only be an artefact. Those chains exist as indivitual functional units.
Chain A of arylformamidase
The red molecule in the middle is an unknown ligand containing a ring composed of 9 oxygen molecules. The green sphere is a chloride ion.
The protein backbone is coloured by conformation type:
Turn - blue
Coil- pink
Helix- green
strand- purple
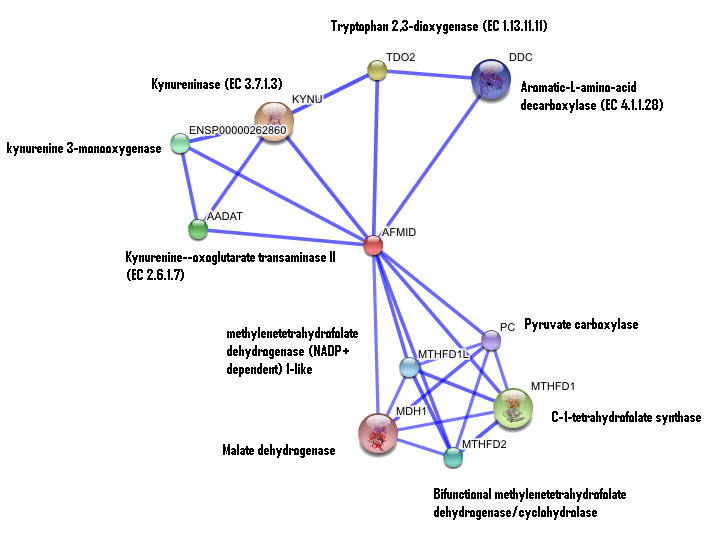
Interaction of human arylformamidase (AFMID) with other proteins
The interaction between the proteins have been determined from curated STRING database (significant score). However there is no significant evidence for:
1- Neighborhood in the genome
2- Gene fusions
3- Cooccurence across genomes
4- Co-Expression
5- Experimental/Biochemical data
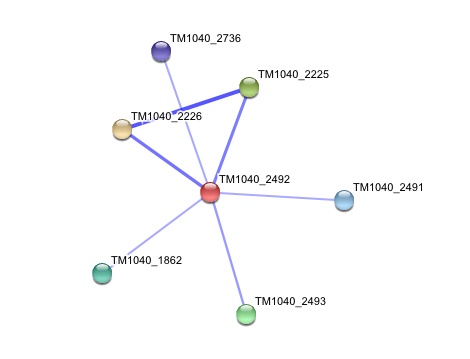
Interaction of Silicibacter Sp. arylformamidase (AFMID) with other proteins
TM1040_2226 Tryptophan 2,3-dioxygenase (279 aa)
TM1040_2225 Kynureninase (396 aa)
TM1040_2493 Succinic semialdehyde dehydrogenase (490 aa)
TM1040_1862 Hypothetical protein (212 aa)
TM1040_2491 Creatinase (402 aa)
TM1040_2736 Transketolase, putative (794 aa)
There is no significant evidence for these interactions (score= ~0.5)
DALI OUTPUT
The DALI tool produces proteins that are structurally similar to the protein of interest.
The search result showed similarities to mostly carboxylesterases/hydrolases. Hence there is strong evidence that our protein might also be a carboxylesterase.
Metagenomic Archea Carboxylesterase (Chain A ONLY)
PDB link title
Note: Chain B not shown
Archaeoglobus fulgidus Carboxylesterase (Chain A ONLY)
File:Carboxylesterase (archaeon).txt
PDB link title
Note: Chains B, C & D not shown
Both of the above Archaeal carboxylesterases' chains exist as monomers (from literature). Hence it is expected that our protein exists as a monomer but during crystalization it interacts with its chains.
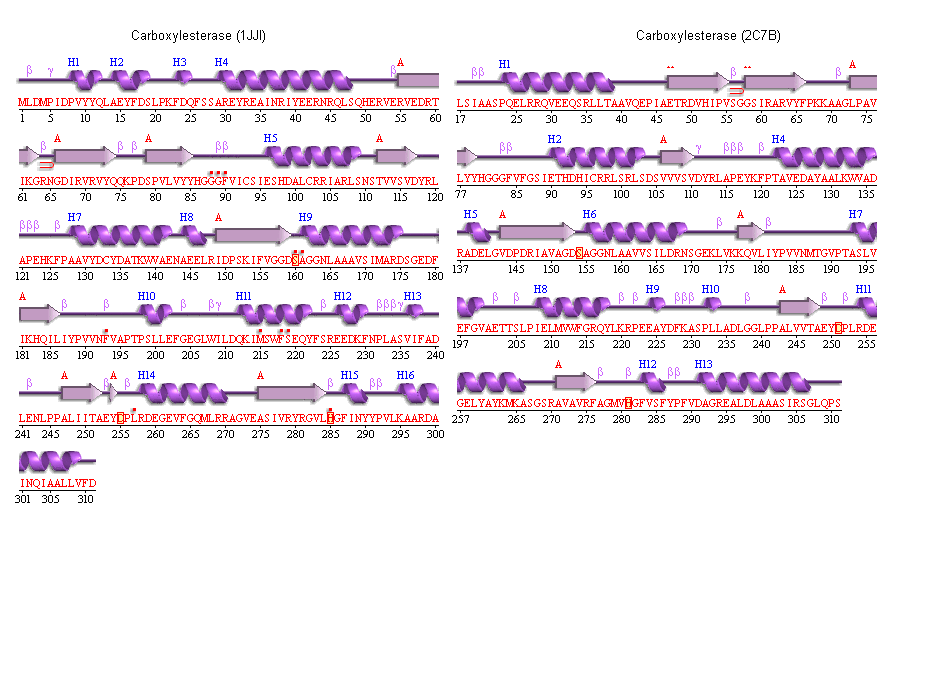
Secondary structure analysis
PDBSum output for arylformamidase
PDBSUM [1]
Archeon Carboxylesterase secondary structure
The conservation of the ser/his/asp catalytic triad
Yellow indicates conservation
Blue indicates semi-conservation
Figure: The catalytic triad
The above image shows the conserved residues of the catalytic triad in arylformamidase, with the unknown ligand (Blue) protruding from a surface groove. The residues are serine 136, Histidine 241 and Glutamate 214. Note: The actual residue numbers are n+1
Image generated using Pymol
Figure: The conserved residues of arylformamidase
The blue region shows the residues conserved among species. It is mostly around the unknown ligand. The conserved residues were obtained from observing the clustal alignment.
Image generated using Pymol
Figure: The catalytic triad
The above image shows the distance between the catalytic triad conserved residues and how each amino acid is linked to a turn region.
From PDB ProteinWorkshop 1.5
Figure: The conserved catalytic triad in Metagenomic Archea Carboxylesterase (PDB ID 2C7B)