Arylformamidase Results
Structure
Arylformamdiase biological structure
The functional biological structure of arylformamidase is assumed by PDB to be a monomer (see figure 2) even though the "whole" protein is shown to be interacting with chains A, B, C and D. The unknown ligand is shown in red and is composed of nine oxygen molecules.
Arylformaidase interactions
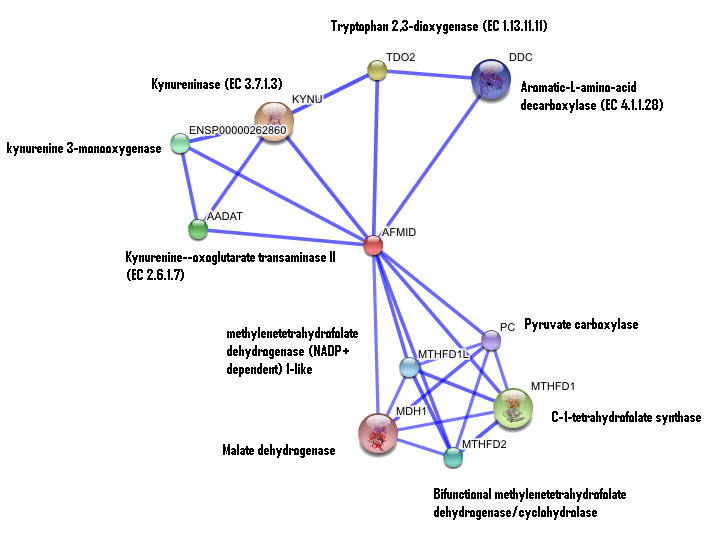
STRING curated database showed that human arylformamidase significantly interacted with a number of proteins. However incidence of any of those proteins/genes to occur repeatedly in close neigbourhood in the genome is not significant. So is their co-occurence (i.e. absence of linked orthologous groups across species) and co-expression across the genome.
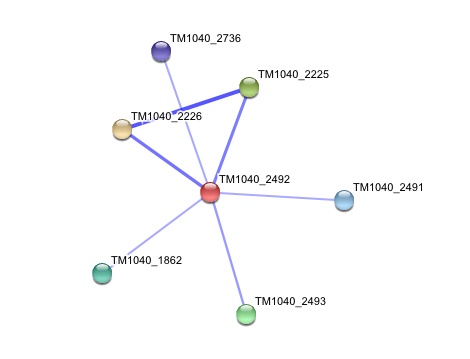
The prokaryotic arylformamidase showed no significant interaction with any of the proteins listed below (score ~0.5)
TM1040_2226 Tryptophan 2,3-dioxygenase (279 aa)
TM1040_2225 Kynureninase (396 aa)
TM1040_2493 Succinic semialdehyde dehydrogenase (490 aa)
TM1040_1862 Hypothetical protein (212 aa)
TM1040_2491 Creatinase (402 aa)
TM1040_2736 Transketolase, putative (794 aa)
Similar strucutures
The DALI tool produces proteins that are structurally similar to the protein of interest. The search result showed similarities to mostly carboxylesterases/hydrolases. Hence there is strong evidence that arylformamidase might also be a carboxylesterase. File:DALI RESULT.txt
The first significant hit from DALI was a metagenomic Archea carboxylesterase. The structure of carboxylesterase shows absence of ligands.
The second significant hit was the carboxylesterase of Archaeoglobus fulgidus. The ligand is present in this figure.
File:Carboxylesterase (archaeon).txt PDB
Both of the above Archaeal carboxylesterases' chains exist as monomers (from literature). Hence it is expected that our protein exists as a monomer but during crystalization it interacts with its chains.
Secondary structure analysis
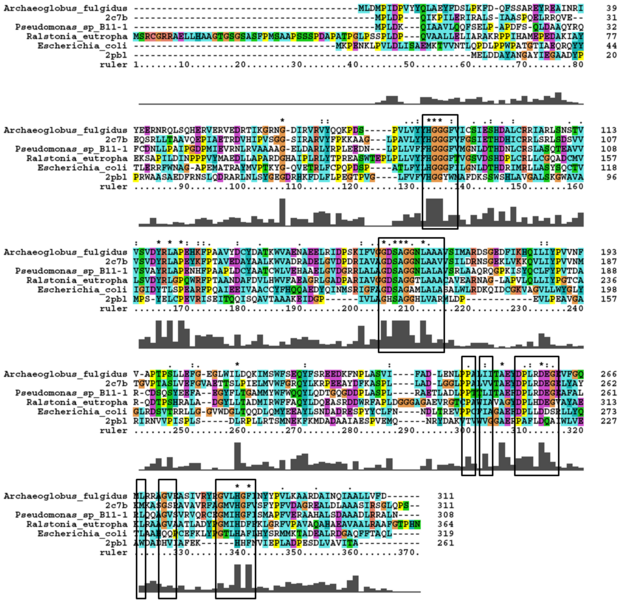
Analysis of arylformamidase's secondary structure with the Archeal carboxylsesterases showed the conservation of the order of occurrence of different conformational types. For instance in all three proteins, the first conformation type is a helix and then three beta strands followed by a helix and so on.
The catalytic triad structure
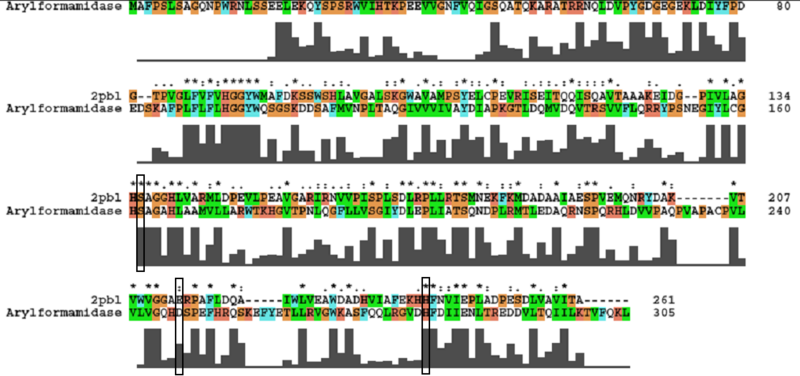
A number of carboxylesterases perform their hydrolysis function using specific catalytic residues. The clustal alignment showed the conservation of the archeal carboxylesterases catalytic triad in arylformamidase. The residues are Serine (Ser) 137, Glutamate (Glu) 215 and Histidine (His) 242. Residues Ser and His were fully conserved whereas E was semi-conserved. Using the human arylformamidase it was observed that Aspartate (asp) was used for eukaryotes instead of Glu, which is used for prokaryotes.
Other conserved/semi-conserved residues were annotated on the structure of arylformamidase. The blue region in the below structure shows how they all are around the catalytic triad and the unknown ligand. This clearly shows their importance for the function of the protein as they have resisted mutations.
The distance between the catalytic triads can be seen in figure 11. Each of the residues are liked to a turn region. This catalytic triad as stated before is also conserved in the Metagenomic Archea Carboxylesterase (PDB ID 2C7B) and the Archaeoglobus fulgidus Carboxylesterase (PDB ID 1JJI). More so the catalytic triad in Archaeoglobus fulgidus Carboxylesterase is very close to the ligand (see figure 12).
Function
The most similar sequence with functional information available was that of an arylformamidase isolated from the liver of Mus musculus (see figure ...). A functional analysis of this protein has been performed identifying a catalytic triad using site-directed mutagenesis (Pabarcus et al. 2007). Conservation of this catalytic triad with 2pbl was assessed. Both residues S162 and H279 were found to be conserved in relatively conserved regions of the alignment. However, D247 had undergone a semi-conservative substitution. These residues correlated to S136, E214 and H241 of 2pbl which were subsequently located on the tertiary structure and determined to be sufficiently proximal to one another for catalysis (see figure...).
most similar structure - catalytic triad, structure with highlighted
2pbl was found to share most structural similarity with a thermostable carboxylesterase from an uncultured archaeon (PDB ID: 2c7b; see figure ...). 2c7b shares a 16% sequence identity with 2pbl. From its structure, a catalytic triad has been identified (how?). To substantiate any functional similarity between 2pbl and 2c7b, conservation of the 2c7b catalytic triad was analysed (see figure ...). All three residues were found to be conserved, though H... and E... were found to match is less conserved regions.
Sequence & Homology
Figure 1 shows that the query sequence "Arylformamidase" grouped with bacterial sequences, shown cloured in Blue. The bootstrap values reveal low confidence with many of the nodes occurring lower down on the phylogenetic tree revealing a possible explanation for certain closely related species to be grouped into separate clades. However, despite low bootstrap scores, the grouping does reliably separate prokaryotes from eukaryotes and the eukaryotes themsselves are clearly distinguished between yeasts and moulds (shown in Green), plants (Dark Green), invertebrates (Orange) and vertebrates (shown in Red).
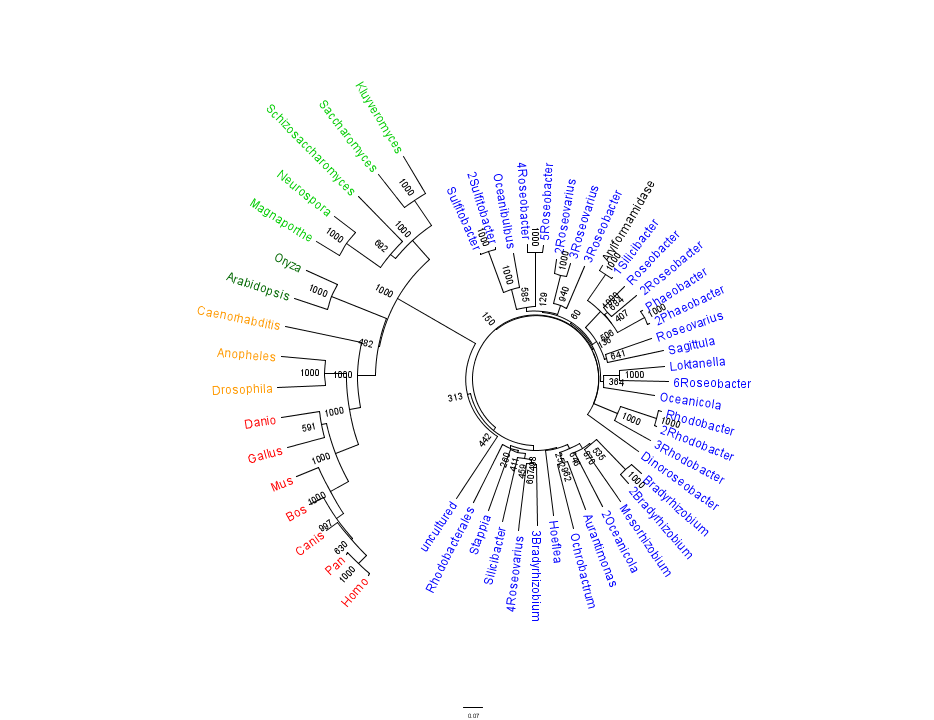
To further elucidate the phylogeny of the Arylformamidase protein, top scoring matches of bacterial homologues were appended with top scoring matches of eukaryotic homologues. Figure 2 is largely consistent with traditional taxonomic groupings of organisms. Specifically, it reveals greater statistical confidence in the separation of prokaryotes (Blue and Green) and eukaryotes (invertebrates are shown in Orange; vertebrates are in Red).
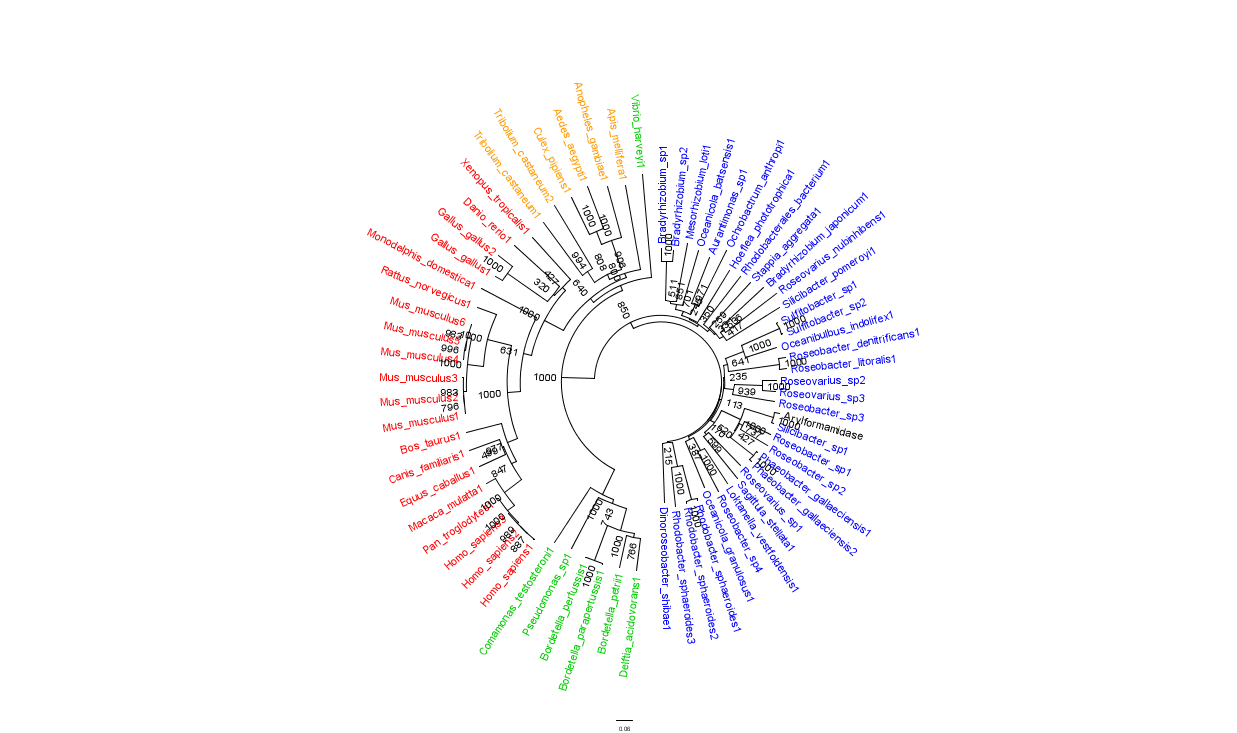
In general, members of the same genus have been grouped together on these phylogenetic trees with some notable exceptions. For instance, Silicibacter, the species from which we derived our protein, occurs on disparate branches of the tree.