DAP discussion
2ijz-A in comparison to 1vhe-A
YsdC from B. subtilis is a putative deblocking aminopeptidase from the M42 family. This gene is conserved in a number of thermophiles, archaea and pathogenic bacterial species. Only one metal cation was seen bound in the active site, defined by residues H68, D182, E214, E215, D237, and H325; a second cation was not observed but two divalent metal cations are probably required for activity. It was modeled as zinc in the structure, but the anomalous signal suggests that it is probably not zinc. Mutation of the aspartic or glutamic acid residues has been shown to have an adverse effect on the function of an aminopeptidase from Pyrococcus horikoshii,38 which requires two cobalt cations for activity. An unusual cis-peptide bond is found between D182 and N183, highlighting its role at the active site. There is one ysdC molecule per asymmetric unit in the crystal, but the protein forms a dimer with a symmetry related molecule, burying 2700 Å2 in surface area, predominantly at the smaller dimerization subdomain (J. Badger Et.AL. 2005).
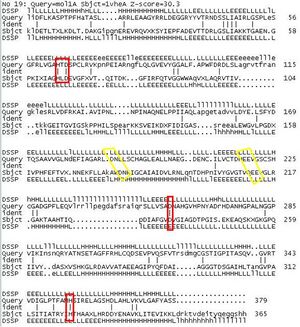
From the Structural comparison of the results seen in the DALI comparison, functional histidines at positions 82 and 401 are seen to be conserved in 1vhe-A and also in the two other proteins that have been marked out earlier on in our DALI search. As we know protein folding differs from protein to protein and structural alignment only gives a rough gauge to how similar the alignment is. As we can see in Fig X, the conserved area between 2ijz and 1vhe is marked with red boxes. Limitations in DALI software often exists, for example as shown in the highlighted yellow boxes although there is clear sequence homology, it does not show any alignment. Therefore from there we can deduce results from the literature paper by J, Badger et al.,2005 that only one metal cation was seen to be bound on the active site which is modeled to be zinc but anomalous signal suggests otherwise. Futhermore, the unusual cis-peptide bond found between D182 and N183 in 1vhe and D180 and H181 in 2ijz, highlights the role at the active site too.
Aspartyl aminopeptidase (DAP) is defined as having a preference for N-terminal aspartyl and glutamyl residues but is distinct from glutamyl aminopeptidase. It is expressed in all tissues at a relatively high level representing >0.1% of soluble protein in cells, Wilk et al. (1998)[1]. It is also expressed at fairly equal levels throughout human tissue; this indicates a basic role in protein metabolism within cells.
Sequence analysis of the protein taken from pseudomonad aeruginosa places it in the M18 family of the MH clan of cocatalytic metallopeptidases. It has been classified as aspartyl amino peptidase and its crystal structure was resolved.
Very few aspartyl aminopeptidases have been characterised to date so little is known of the function of these enzymes. They are believed to be widely distributed in eukaryotes and prokaryotes. The function is to cleave amino acid residues at the N-terminus of unblocked peptides. To date yeast, human and the malarial parasite aspartyl aminopeptidase have been extracted and characterised. Genome sequencing has identified several other homologues however they are yet to be confirmed experimentally.
Many families of proteins within MH clan of metallopeptidases are characterised by the zinc binding motifs such as HEXXH however it is now known that no such motif exists for M18 family proteins, Yokoyama et al.(2006)[2]. Due to very little knowledge about that active sites of these proteins its catalytic centres are characterised based on sequence homology which has yielded a small selection of highly conserved His, Glu and Asp residues which are known to be involved in zinc binding. Two of three His residues that are considered to be fully conserved across M18 members are thought to be involved in the coordination of Zn2+ ions at the active site as mutation of these residues results in abolition of enzymatic function, Wilk et al. (2002).
To identify or predict the structure, function and evolutionary history of DAP, many tools were utilised. P. aeruginosa DAP is composed of 12 identical subunits each with 428 residues. A DALI search was able to identify structural similarities with the current DAP chain-A and other aminopeptidase moleules, see figure 3.4.
To predict the function of P. aeruginosa DAP, an InterPro scan identified the sequence as Aminopeptidase I zinc metalloprotease (M18). It listed 6 motifs of commonly conserved regions within the family. Based on the two papers by Wilk et al. (1998) and Wilk et al. (2002)[3] Glu, Asp and His residues that were conserved and predicted for catalytic function were identified in the P. aeruginosa DAP. These residues mostly fell into these nominated motif regions indicating that the active sites were present there. A structural alignment between mouse and human with P. aeruginosa DAP showed that all the nominated residues were fully conserved between the species, making P. aeruginosa a good candidate to study DAP in a bacterium. A CluSTr analysis of the sequence was more accurate and figures 1.5-7 show the results. It predicted a probable M18 family aminopeptidase 2 and gave three potential zinc binding residues His 82, 156 and 401 which correspond directly to the human residues predicted in the aforementioned paper.
Analysis of chain-A structure reveals that the residues of the predicted active sites cluster together figure 4.3. This indicates that the predictions are correct however analysis of the whole structure is needed to confirm this.
Viewing the current DAP in PyMoL reveals a dodecameric (12 subunits) tetrahedral shape, this has been identified previously in other members of the MH clan such as the FrvX homolog, glutamyl aminopeptidase, in P. horikoshoii, Russo & Baumann (2004)[4]. This protein features a catalytic zinc centre where substrates can access the active site through four channels in the centre of each triangular face of the dodecameric tetrahedral structure. This ensures that small or completely unfolded proteins can act as substrates.
One of the problems with determining the function of a crystallised protein is whether it dissociates in vivo to perform its biological function or whether the crystal structure is maintained and is necessary for activity. The paper by Wilk et al. (2002), provides evidence that DAP retains a three dimensional structure as it proposes that His352 is important for subunit-subunit interactions. Mutation of this residue dissociates the enzyme, and catalytic function is lost implying that oligomeric DAP is catalytically active. This paper was however published before the crystal structure for DAP was resolved and it states that assignment of function to the histidines analysed awaits the solution of the crystal structure. Using PyMoL to analyse the named His352 residue or His313 in P. aeruginosa in the crystal structure of DAP shows that the residue is located between adjacent subunits figure 4.4 indicating a structural role.
Similarly Histidines 349, 359 & 363 in the human homologue were experimentally shown to reduce kcat but not km so the paper states these also may contribute to stability. However structural analysis of Histidines 310, 320 and 324 in P. aeruginosa show that this cluster is located in three sites around a single channel in each face of the tetrahedral structure figure 4.5. This indicates a role in guiding the substrate through the channel to the active site. So the reduction in kcat is probably due to the reduced likelihood of the substrate coming into contact with the active site rather than destabilising the structure.
To verify the above assertion the nominated active sites His82 and His401 were analysed in the crystal structure and it was found that they were distributed in the internal part of the structure figure 4.6. This indicates a similar system to that of the FrvX homolog in P. horikoshoii mentioned above.
Knowledge regarding this protein has possible applications in a variety of areas in relation to human health. One paper identified DAP in the malarial parasite Plasmodium falciparum, mutation of certain residues in DAP is shown to be lethal and this could be useful in producing a new anti-malarial drug as there is increasing resistance to the drugs currently available, Teuscher et al. (2007).
Also it has been suggested that DAP could play a role in Alzheimer’s disease. As it facilitates the removal of an aspartyl residue from β-amyloid, a reduction in this process can lead to β-amyloid deposition, Wilk et al. (1998).
Aspartyl aminopeptidase is widely presented in many organisms. Through a Multiple Sequence Alignment (MSA) with a non-redundant database, many homologous sequences could be matched with our query sequences. Many databases entries which matched were derived from genome sequencing and the enzymatic activities have not been determined but it is likely that they are also aspartyl aminopeptidases.
From the phylogenetic trees shown, they could be interpreted such that the organisms matched could be classified using the two-empire and five-kingdom system. From figure 2.7, several bootstrap values could be found to be lower than 75%, but this could be due to software limitations, for example; the rightful pseudomonad fluorescens was grouped along with its similar pseudomonad groups, and yet its branching was <75%.
Concluding the information provided by the phylogenetic trees, it could be deduced that DAP genes have always been vertically transferred and not laterally. This is true considering the fact that the function of the protein is ubiquitous and essential in most cells.
[5] Back to main page, aspartyl aminopeptidase.